Please note that new patient recall registrations closed effective December 31, 2024. Registered patients have until June 30, 2025 to make any required elections in the recall registration portal at which time no further input can be made. By end of July, Philips Respironics will satisfy all available direct-to-patient CPAP and BiPAP orders based on information provided in the portal. Effective August 1, 2025, Philips Respironics will cease to provide the recall registration and remediation reports.
Philips Registration and Remediation Report Discontinuation

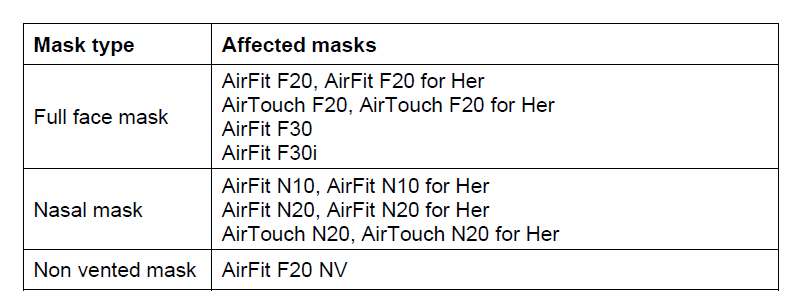
Urgent Field Safety Notice ResMed Masks with Magnets - Potential Magnetic Interference with Certain Medical Devices
ResMed is providing you with important information regarding our masks with magnets, which may interact with some implants or certain medical devices. It is important that you understand the information contained in this letter, take action if necessary, and keep it for future reference.
In response to new information from latest industry practices and global safety data, ResMed is updating the Contraindications and Warnings in our User Guides to instruct patients on the safe use of ResMed masks with magnets.
- Contraindications: conditions under which the mask is not to be used.\
- Warnings: identify a possible hazard and provide information on how to safely use the mask.
General Product Description
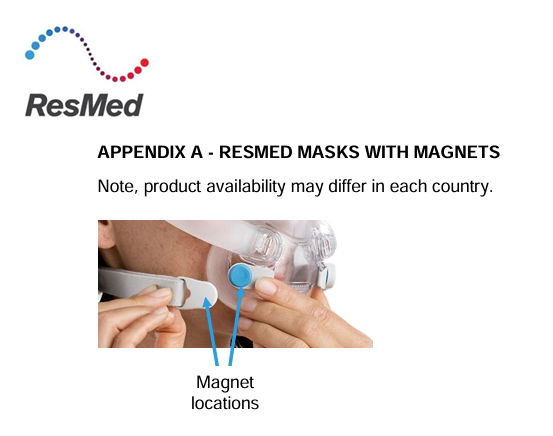
These masks are used to deliver air to a patient from a positive airway pressure (PAP) device. Magnets are used in some ResMed masks to provide a simple and easy way for patients to attach and detach the headgear to the mask frame when regularly fitting the mask for use. This can be notably beneficial to patients with disabilities, including those facing dexterity or vision impairment.
The following ResMed masks contain magnets, please note that mask availability may differ in each country.
PHILIPS RESPIRONICS RECALL
“Philips Respironics Recalls Certain Reworked DreamStation CPAP, BiPAP Machines for the Risk They May Deliver Inaccurate or Insufficient Therapy” – FDA.gov
Reason for Recall
Philips is recalling certain reworked Philips DreamStations because some devices were assigned incorrect or duplicate serial numbers during initial programming. This duplication can cause therapy to be delivered using the wrong prescription or factory default settings. Additionally, it may fail to deliver any therapy at all. There is no warning or indication to the user that the DreamStation is not working the way the doctor intended or prescribed.
Incorrect therapy or therapy failure may lead several health conditions such as respiratory failure, heart failure, serious injury, and death.
Philips has received 43 complaints about this issue. There are currently no reported injuries or deaths.

PHILIPS RESPIRONICS MEDIAL DEVICE CORRECTION
Philips Respironics Sleep and Respiratory Care
The following Philips Respironics patient interface devices (face and nasal masks) – Amara View Minimal Contact Full-Face Mask, DreamWear Full Face Mask, DreamWisp Nasal Mask with Over the Nose Cushion, Wisp Nasal Masks and Wisp Youth Nasal Masks, and Therapy Mask 3100 NC/SP – contain magnets.
- Amara View Minimal Contact Full-Face Mask
- DreamWear Full Face Mask
- DreamWisp Nasal Mask with Over the Nose Cushion
- Wisp Nasal Masks and Wisp Youth Nasal Masks
- Therapy Mask 3100 NC/SP
This Urgent Medical Device Correction is intended to inform you that Philips Respironics is updating its existing ‘Contraindications’ and ‘Warning’ of the above masks with magnets to the below.
Contraindication: Use of the mask is contraindicated for patients and their household members, caregivers, and bed partners that may be in close vicinity to patients using the masks, that have implanted devices that may be affected by magnets, including but not limited to:
- Pacemakers
- Implantable cardioverter defibrillators (ICD)
- neurostimulators
- Magnetic metallic implants/electrodes/valves placed in upper limbs, torso, or higher (i.e. neck and head)
- CSF (cerebral spinal fluid) shunts (e.g., VP (ventriculo peritoneal) shunt)
- Aneurysm clips
- Embolic coils
- Intracranial aneurysm intravascular flow disruption devices
- Metallic cranial plates, screws, burr hole covers, and bone substitute devices
- Metallic splinters in the eye
- Ocular implants (e.g., glaucoma implants, retinal implants)
- Certain contact lenses with metal
- Implants to restore hearing or balance that have an implanted magnet (such as cochlear implants, implanted bone conduction hearing devices, and auditory brainstem implants)
- Magnetic denture attachments
- Metallic gastrointestinal clips
- Metallic stents (e.g., aneurysm, coronary, tracheobronchial, biliary)
- Implantable ports and pumps (e.g., insulin pumps)
- Hypoglossal Nerve Stimulators
- Devices labeled as MR (magnetic resonance) unsafe
- Magnetic metallic implants not labeled for MR or not evaluated for safety in a magnetic field
Warning: Magnets with a magnetic field strength of 400 mT are used in the mask. With the exception of the devices identified in the contraindication, ensure the mask is kept at least 6 inches (approx. 15.24 cm) away from any other medical implants or medical devices that can be impacted by the magnetic fields to avoid possible effects from localized magnetic fields. This includes household members, caregivers, and bed partners that may be in close vicinity to patients that use the masks.
For a full list of Products affected including Mechanical Ventilators, please visit their website: Philips.com.
For more information on the Mask Recall from Philips, please visit: https://www.fda.gov/medical-devices/letters-health-care-providers/certain-philips-respironics-masks-bipap-cpap-machines-recalled-due-safety-issue-magnets-may-affect

What is a Contraindication versus a Warning?
A contraindication is a condition under which the device should not be used because the risk of use clearly outweighs any possible benefit. A warning alerts the patient about a situation which, if not avoided, could result in death or serious injury. Additionally, a warning may also describe potential serious adverse reactions and safety hazards. In short, a contraindication tells the user when a device should not be used, and a warning tells the user how to avoid sources of harm in the use of the device.
What the problem is and under what circumstances it can occur
The affected masks contain magnets which can potentially affect the functioning and/or induce the movement/dislocation of medical implants or medical devices that can be impacted by the magnetic fields.
What should I use instead of these products?
- Amara View Minimal Contact Full-Face Mask and Wisp/Wisp Youth have non-magnetic headgear clip replacement parts that can be used in place of the magnetic headgear clips.
- Amara View Minimal Contact Full-Face Mask with non-magnetic headgear clips is an alternative to DreamWear Full Face.
- DreamWear under-the-nose nasal mask, Wisp with non-magnetic headgear clips, and Pico are alternatives to DreamWisp Nasal Mask with Over the Nose Cushion.
- DreamWear under-the-nose nasal mask is an alternative to Therapy Mask 3100 NC.
- DreamWear Silicone Pillows, Nuance, and Nuance Pro are alternatives to the pillows in Therapy Mask 3100 SP.
Describe the hazard/harm associated with the issue
With the exception of the devices in the contraindication, if the mask magnets are placed less than 6 inches (approx. 15.24 cm) away from a metallic implant or device the magnets may cause the device to not perform as intended, which may result in a serious injury.
There have been fourteen (14) reports of patients suggesting that the mask magnets have impacted their medical devices which include: pacemaker interference, pacemaker failure leading to replacement, need of shunt adjustment, resetting of automatic implantable cardioverter defibrillator (AICD), seizures, defibrillator shutting off periodically, arrhythmia, irregular blood pressure, change in heartbeats, and cognitive issues.
I have an implant/medical device and still want to use the mask with magnets OR my physician says I can continue to use a mask with magnets; what do I do?
You should discuss the updated Contraindication and Warning with your physician.
I believe I have had adverse events or harm because of the magnets in my mask; what do I do?
STOP using the affected mask and contact a healthcare provider immediately. Report the event to the FDA MedWatch: The FDA Safety Information and Adverse Event Reporting Program, either online, or by regular mail, or by fax.
Affected products and how to identify them
Figures 1 5 shows the images of the affected masks and Figure 6 (next page) contains their part numbers. The magnetic headgear clips on these masks are circled. Use the below images (Figures 1-5) and the Part Numbers (see Figure 6) in this letter, to determine if your or your patient’s mask also uses magnetic headgear clips. These masks are intended to provide an interface for application of CPAP or bi-level therapy to patients.

Actions to be taken by patient to prevent risks
STOP using the affected mask, if the implant/medical device is contraindicated against the mask magnets. Patients should consult their physician immediately to determine if another mask can be used for their therapy. In the interim, switch to a non-magnetic mask if available, for continued therapy. Properly dispose of the mask that has magnets after an alternative is obtained.
If you, household members, caregivers, and bed partners who may be in close vicinity to you, do not have implanted medical devices, or metallic splinters in the eyes, then no action is needed.
Household members, caregivers, and bed partners with a medical implant/device must ensure the mask is kept at least 6 inches (approx. 15.24 cm) away from the medical implant(s)/device(s).
Contact Philips Respironics customer service for more information on non-magnetic mask options.
Actions planned by Philips to correct the problem
Philips Respironics is updating its existing Contraindication and Warning as provided in this correction letter.
If you need any further information or support
If you need any further information or support concerning this issue, please contact your local Philips representative. For general issues or concerns, contact the Philips Customer Care Solutions Center at +1-800-345-6443.
PHILIPS RESPIRONICS RECALL
For all our patients and CPAP users, we want to inform you that on June 14, 2021, Philips Respironics has recalled various models of CPAPs, BiLevel PAPs, and ventilators (including non-invasive and invasive ventilation).
The recall was initiated due to two issues related to the polyester-based polyurethane (PE-PUR) sound abatement foam used in these devices.
“At that time, out of an abundance of caution and based on available information, Philips advised of potential health risks related to sound abatement foam used in specific Philips Continuous Positive Airway Pressure (CPAP), BiLevel Positive Airway Pressure (BiLevel PAP) devices, and Mechanical Ventilators.”
– Philips.com
Possible health risks include exposure to degraded sound abatement foam, for example caused by unapproved cleaning methods such as ozone, and exposure to chemical emissions from the foam material. Philips says potential health risks include that the foam may degrade into particles that may enter the device’s air pathway and be ingested or inhaled by the user, and the foam may off-gas certain chemicals.
High heat and high humidity environments may also contribute to foam degradation in certain regions. Philips has determined that the foam may degrade under certain circumstances, influenced by factors including use of unapproved cleaning methods, such as ozone), and certain environmental conditions involving high humidity and temperature.
The potential risks of degraded foam exposure include:
- Irritation (skin, eye, and respiratory tract), inflammatory response, headache, asthma, adverse effects to other organs (e.g. kidneys and liver) and toxic carcinogenic affects.
- To date, Philips Respironics has received complaints regarding the presence of black debris/particles within the airpath circuit (extending from the device outlet, humidifier, tubing, and mask). Philips also has received reports of headache, upper airway irritation, cough, chest pressure and sinus infection.
The CPAP and BiLevel PAP Devices that are affected by the recall are:
- E30 Continuous Ventilator
- DreamStation ASV
- DreamStajtion ST, AVAPS
- SystemOne ASV4
- C Series ASV, S/T, AVAPS
- OmniLab Avanced Plus In-Lab Titration Device
- SystemOne (Q Series)
- DreamStation CPAP, Auto CPAP, BiPAP
- DreamStation GO CPAP, APAP
- Dorma 400, 500 CPAP
- REMStar SE Auto CPAP
For a full list of Products affected including Mechanical Ventilators, please visit their website: Philips.com.
For more recall information on the PAP devices: www.philips.com/src-update


